SATS
Southern African Transplantation Society
Position Statement
Social Media Donor 2025
Introduction
- Social media has proven to be a powerful tool for increasing awareness about organ donation and transplantation, reaching millions of people rapidly and effectively. Campaigns and personal stories shared on platforms like Facebook, Twitter, and Instagram can dispel myths, provide accurate information, and encourage individuals to register as organ donors.
- The use of social media in organ donation raises significant ethical issues, including concerns about privacy, coercion, and the potential for misinformation or exploitation. Without clear policies, individuals may inadvertently share sensitive personal information or feel pressured to donate due to public solicitation.
- Not all patients have equal access to social media or the skills to use it effectively, which can create disparities in who benefits from online organ donation campaigns. A policy can help ensure that guidance is provided to minimize these disparities and promote fair access to organ donation opportunities.
- Transplant centres and hospitals face challenges in guiding patients on how to use social media safely and effectively to find living donors. Policies can offer step-by-step guidance on what information is appropriate to share, how to protect privacy, and how to avoid legal or ethical pitfalls such as coercion or undue influence.
- Social media communities are arranged in many ways and without standardization, raising concerns about transplant candidates and potential donors' quality of care. Currently, no national ethical guidelines have been developed regarding the use of social media to foster organ transplantation. We need an ethical framework to guide transplant stakeholders in using social media for public and patient communication about transplantation and living donation, and to offer recommendations for transplant clinical practice.
- Professional societies (SATS, SATCS) can play an important and influential role in recommending that transplant hospitals raise awareness, informing their patients, and staff about risk and benefits of using social media when seeking a living donor organ for transplantation.
- Transplant hospitals that do not use or encourage transplant candidates' use of social media, may inadvertently create more disparities for transplant access. Transplant hospitals can reduce potential disparities in access to transplantation by encouraging the ethical use of social media.
Purpose
- The primary purpose of a social media transplant policy is to provide clear guidance for members of the transplant community-including professionals, patients, and organisations-on how to engage responsibly and effectively with persons seeking transplant that have met on social media platforms. This ensures that interactions support the mission of increasing organ donation and transplantation while safeguarding against potential risks such as misinformation, privacy breaches, and ethical concerns.
- The policy establishes boundaries to protect patient confidentiality and donor privacy. It addresses ethical issues such as coercion, undue influence, and the risk of exploitation or commercialisation of organ donation.
- By setting standards for content and conduct, the policy helps maintain public trust and ensures that social media activities align with professional and legal standards.
- To ensure that there is standardisation, compliance and clear guidelines related to the transplantation of patients and or individuals who have secured a potential living donor on social media.
- To ensure that there is standardisation, compliance and clear guidelines related to the transplantation of patients and or individuals who have identified a potential living recipient on social media. (Advertised by the person seeking assistance for a transplant).
- Outline clear guidelines and procedures for using social media platforms to ethically and effectively promote and Facilitate Altruistic organ donation.
- Ensures standardised approach to awareness and education – accurate respect and inspiring content is shared to raise public awareness. And importance of altruistic organ donation.
- Directs engagement with Donor's recipients and Transplant teams.
- To ensure that the processes comply with National Laws, Medical Ethics, organisational policies and The Declaration of Istanbul - related to organ donation social media communication.
- Potential risks like misinformation, breach of confidentiality, or incentivised donation are managed through clear processes and protocols.
- To ensure that processes policies and procedures are in place to identify coercion and financial incentivisation and to prevent this in all possible instances.
Defining Social Media
- Social media is a collection of web-based technologies that share a user-focused approach to design and function, where users actively participate in content creation. Social media (e.g. blogs, tweets, wikis, crowdfunding and social networks) can accelerate and foster communication and action among large communities across wide geographical locations. Facebook, used by 80% of all internet users, is currently the most popular social media site. Social media also expresses individual and cultural identity. Additionally, deeply felt connections between strangers can form on social media too thereby creating donor-recipient intimacy before face-to-face meetings. Social media has changed our definition of "friend". As a result, transplant candidates may feel more comfortable asking online friends for an organ.
- Currently the transplant community uses social media to promote deceased donor registration and the benefits of deceased donation.
- Transplant programs are increasingly using Facebook to promote and advertise their programs and to provide resources to the public about living donation. Transplant candidates creating personal Facebook pages to identify potential donors was first documented in 2011.
Privacy and Confidentiality
- Maintaining recipient and donor privacy/confidentiality is becoming increasingly challenging with the explosion of social media and news media coverage about organ donation.
- Anecdotal evidence from online transplant lists suggests that recipients commonly seek information about their donors from news sources like online platforms, local TV news and newspaper obituaries.
- Privacy and confidentiality are further challenged by viral social media posts, which are especially persuasive and involve widespread dissemination of the user-intended message through peer-to-peer communication.
- For donors and recipients whose only relationship is "virtual", social media may create a potential power imbalance between the parties, and some capabilities, such as private and direct messaging, may inadvertently create tension. For example, a potential donor may wish to meet a transplant candidate before the transplant, but the feeling may not be mutual.
- The transplant candidate could feel pressured to meet with the donor before the transplant in order to ensure the donation. Social media can therefore create the possibility of a non-directed donation becoming a directed donation.
- Ensure that the Transplant teams working with these candidates protect the identities and medical information of both parties and their families unless they have explicitly consented without coercion to share.
- Individuals must be free to make informed voluntary decisions (Autonomy) about organ donation
- Social media content must never pressure, guilt or manipulate users to become donors.
- Organ donation should be viewed by both parties as a gift not a transaction
- Under all circumstance the transplant teams should ensure that harm is avoided, by preventing exploitation of venerable populations or promotion of unsafe practices
- Potential donors should understand that posting about interest in donation or one's donor evaluation can lead to uninvited public feedback.
Truthfulness
- Truthfulness is a significant ethical consideration given that what transplant candidates reveal about themselves personally or medically on social media may potentially influence who is interested in donating. Transplant candidates may feel compelled to compose their social media profiles in the most positive sounding way to draw more potential donors to their cause.
- Transplant personnel should provide guidelines to transplant candidates stressing the importance of truthfulness in representing oneself in social media profiles, and the potential harm to potential donors of transplant candidates' misrepresentation and embellishment.
Informed Consent, Education, Undue Influence and Coercion
- Social media has the capacity to affect the informed consent process and present added risks to potential donors or transplant candidates.
- The scarcity of organs and transplant candidates' desperate medical needs make stories shared through social media compelling to potential donors and friends or family.
- Facebook posts may present a spectrum of personal and identifying information, from positive to negative, from truthful to embellished.
- Individuals may deliberately enhance their mediagenic profiles to boost interest among followers.
- Therefore, the informed consent process for potential donor or transplant candidates should include discussion of the potential influence of social media on decision making treatment.
- Social media provides a public forum where undue influence, external pressure to donate and coercion may arise to a larger degree, raising greater concerns than for traditional communication.
- Social media posts can reach large audiences, frequently far beyond immediate social circles.
- Feigned interest can contribute to individuals initiating donor evaluation, only to later drop out as the process intensifies which could create false hope for the transplant candidates and undermine the ethics of care.
- Barriers to identifying potential donors include discomfort with initiating discussions, concerns about inducing guilt, or burdening family members and friends and these barriers may also extend to social media.
Equity and Fairness
- No group or individual should be unfairly targeted in donation campaigns, equal action for donation and transplant services is bound by law.
- Transplant hospitals that do not use or encourage transplant candidates' use of social media, may inadvertently create more disparities for transplant access. Transplant hospitals can reduce potential disparities in access to transplantation by encouraging the ethical use of social media.
Definitions
I. Altruism
The selfless concern for the well-being of others, often demonstrated through actions intended to help others without expecting any personal gain, reward, or benefit.
II. Living Recipient
Person who receives an organ or a portion of an organ from a living donor through a surgical transplant procedure. This typically occurs when someone with end-stage organ failure-such as kidney or liver failure-receives an organ from a healthy living person, rather than waiting for an organ from a deceased donor.
III. Social Media
A collection of web-based technologies that share a user-focused approach to design and function, where users actively participate in content creation. Social media (e.g. blogs, tweets, wikis, crowdfunding and social networks) can accelerate and foster communication and action among large communities across wide geographical locations.
Press and Social Media
Important Guidelines
Transplant candidates and potential living donors should be informed to NOT post the following items to social media:
- Personal phone numbers
- Personal email addresses
- Residential addresses
- Family information
- Any other sensitive information
- Inappropriate photographs
Roadmap of the Social Media Donor and Recipient
At any stage of the assessment if candidates are found to be unsuitable the process will be halted and candidates informed.
- South African Transplant centres will need to collaborate on a central register of Social media donors and recipients to ensure that if candidates are declined they do not centre hop.
- Potential Altruistic candidates contact the Transplant Centre or Transplant staff
- Transplant candidate and donor evaluation should address social media risk and uncertainties, and their potential influence on decisions concerning donation.
- Potential donors should understand that posting about interest in donation or one's donor evaluation can lead to uninvited public feedback.
- An appointment for an information session is made for the donor or recipient to ensure a standardised approach to awareness and education – accurate respect and inspiring content is shared to raise public awareness and importance of altruistic organ donation, if candidate is found to be unsuitable, they will be informed and the process halted.
- Basic Physical exam (Assessment) and if suitable will be further referred for MDT assessment
- Candidates seen by the transplant MDT team will need to undergo:
- Psychosocial Screening 1. - to determine suitability
Although the current psychosocial evaluation process already investigates the potential financial motivation to donate, a new assessment question should be tailored to donors identified via social medica, such as "Was there anything mentioned in the post you saw that would indicate you would get some type of reward or incentive for donating to this person?"
- Psychosocial assessment 2. Pre and post Donation at Primary Referral Centre
Although the current psychosocial evaluation process already investigates the potential financial motivation to donate, a new assessment question should be tailored to donors identified via social medica, such as "Was there anything mentioned in the post you saw that would indicate you would get some type of reward or incentive for donating to this person?"
- Psychosocial assessment 3. Alternate transplant Centre and screening tools to support the altruistic intent
Although the current psychosocial evaluation process already investigates the potential financial motivation to donate, a new assessment question should be tailored to donors identified via social medica, such as "Was there anything mentioned in the post you saw that would indicate you would get some type of reward or incentive for donating to this person?"
- Psychosocial Screening 1. - to determine suitability
Social Media Donor and Recipient Pathway
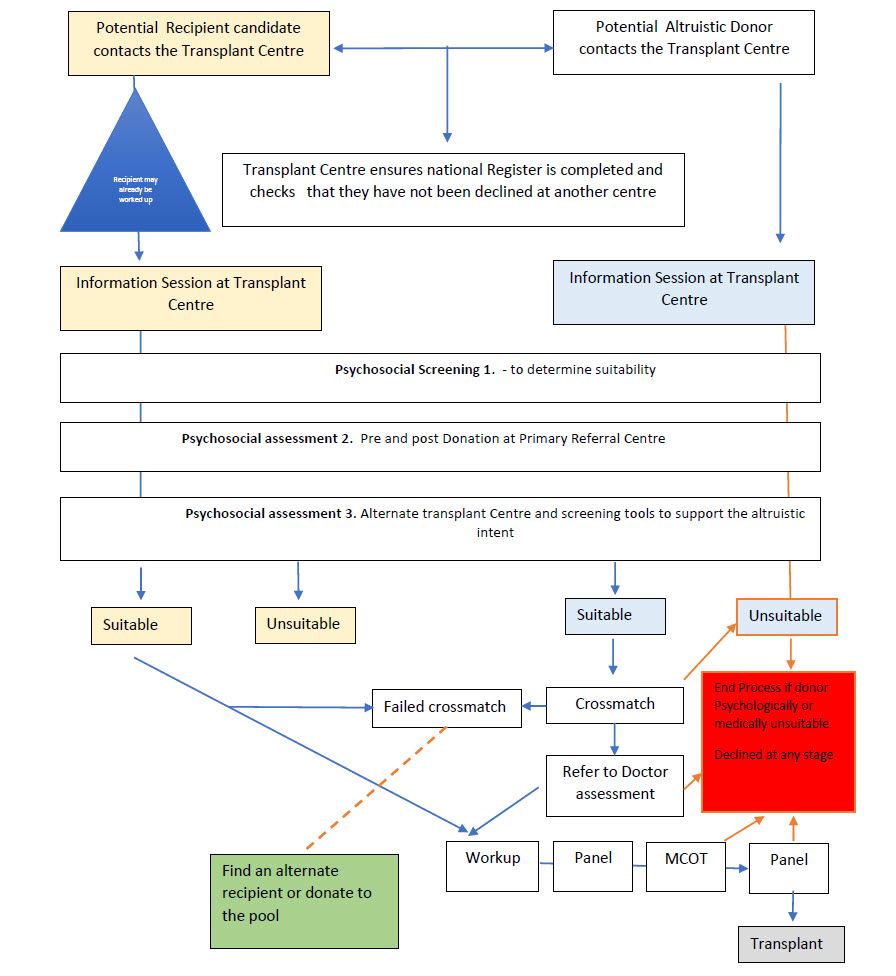
Approval to Proceed or Not to Proceed
- Patient will be presented at a local Transplant Panel meeting for unanimous decision to proceed or not to proceed.
- Referred to a Nephrologist and other MDT members as per work up process if approved.
Panel Approval
- The case needs presentation at Panel to strengthen the application at MCOT or strengthen and document the decision not to proceed.
- Any decision taken will be updated in the National patient registry.
Work up
All Candidates will, if approved, follow the standard ULD workup process and approvals framework
Important Note: It is very important to note that policing transplant candidates online/social media profiles is outside the scope of professional responsibility for transplant personnel.
References
- Henderson ML, Clayville KA, Fisher JS, Kuntz KK, Mysel H, Purnell TS, Schaffer RL, Sherman LA, Willock EP, Gordon EJ. Social media and organ donation: Ethically navigating the next frontier. Am J Transplant. 2017 Nov;17(11):2803-2809. doi: 10.1111/ajt.14444. Epub 2017 Aug 24. PMID: 28744966.
- L. Henderson, K.A. Clayville, J.S. Fisher, K.K. Kuntz, H. Mysel, T.S. Purnell, R.L. Schaffer, L.A. Sherman, E.P. Willock, E.J. Gordon,Social media and organ donation: Ethically navigating the next frontier,American Journal of Transplantation,Volume 17, Issue 11, 2017
- Meena P, Kute VB, Bhargava V, Mondal R, Agarwal SK. Social Media and Organ Donation: Pros and Cons. Indian J Nephrol. 2023 Jan-Feb;33(1):4-11. doi: 10.4103/ijn.ijn_158_22. Epub 2022 Dec 26. PMID: 37197042; PMCID: PMC10185012.
- (Chang A et al. Identifying potential kidney donors using social networking website. Clin Transplant. 2013;27:E320 – E326.)
- Meena P, Kute VB, Bhargava V, Mondal R, Agarwal SK. Social Media and Organ Donation: Pros and Cons. Indian J Nephrol. 2023 Jan-Feb;33(1):4-11. doi: 10.4103/ijn.ijn_158_22. Epub 2022 Dec 26. PMID: 37197042; PMCID: PMC10185012.
- 1 Basu, Gopal1,2; Nair, Sanjeev2,3; Bek, Sibel Gokcay2,4; Dheerendra, Prashant2,5; Penmatsa, Krishnam Raju2,6; Balasubramanian, Karthikeyan2,3; Shingada, Aakash2,7; Conjeevaram, Arvind2,8,*. Social Media and Organ Donation - A Narrative Review. Indian Journal of Transplantation 15(2):p 139-146, Apr–Jun 2021. | DOI: 10.4103/ijot.ijot_138_20
- Roy Allan The role of social media in organ donation advocacy. Department of Sociology, Duke University, Durham, USA. https://www.alliedacademies.org/articles/the-role-of-social-media-inorgan-donation-advocacy-26626.html
- Eckler P, Bolls P. Spreading the virus: emotional tone of viral advertising and its effect on forwarding intentions and attitudes. J Interact Advert. 2011;11:1-11
Document Information
By Mande Toubkin and Ethics working group
South African Transplant Society (SATS)
Contact Information
Dr Priya Walabh
Tel: +27(0)732333155
Email: priya.walabh@wits.ac.za
President of South African Transplant Association (SATS)
Version 1 | 13 July 2025
South African Transplant Society
COVID-19 VACCINATION
Position Statement on COVID-19 Vaccination in South Africa
The South African Transplantation Society (SATS) recommends that all patients for organ transplantation, healthcare professionals in transplant teams, and family members be vaccinated against COVID-19.
We recognize and advocate for the use of vaccines in the prevention of COVID-19 infections. There is clear scientific evidence for the use of vaccinations to reduce the risk of both hospitalization and death in COVID-19 infections, with the benefit of vaccination far outweighing its risk.
The mortality rate for transplant recipients from COVID-19 is reported to be 20%, which is 10-fold higher than the general population (1), due to their need for lifelong immunosuppression medication.
With such a scarce resource such as organs for transplantation, there is an ethical obligation to ensure that the gift of donation is used in the best way possible and for maximum benefit. Given the ongoing extreme shortage of donors, transplant centers will consider COVID-19 positive deceased donors as potential donors given early favorable results of transplants from selected COVID-19 positive donors. (2)
As such, transplant centers require patients to demonstrate adherence to optimum medical therapy prior to surgery and that patients undertake to continue such therapy after transplantation. Adherence to best medical therapy (e.g., for diabetes and hypertension), being fully vaccinated including booster doses (e.g., against hepatitis B), abstinence from alcohol consumption (in cases of alcoholic liver cirrhosis) are already established as routine in a transplant assessment and work-up.
Transplant centers should appropriately counsel patients and their donors about their need for COVID-19 vaccination. They should play an active role in ensuring that misinformation and vaccine hesitancy are appropriately addressed as part of that work-up. For children receiving transplants, it is important that family members are also fully vaccinated.
Further information:
Currently in South Africa there are two SARS-CoV-2 vaccines being used in the national vaccine program: the Pfizer-BioNTech and the Johnson & Johnson vaccines. The Pfizer-BioNTech vaccine is an mRNA vaccine that is given as two doses separated by 21 days. The Johnson & Johnson vaccine is an adenovirus-vectored DNA vaccine given as a single dose.
Both were evaluated in randomized controlled trials involving over 40,000 participants to evaluate efficacy and safety before being used in vaccine programs. In these trials, the Pfizer-BioNTech vaccine resulted in a 95% reduction in COVID-19 infections (3). A similar reduction in cases of severe COVID-19 was observed. The Johnson and Johnson vaccine resulted in an 85% reduction in severe-critical COVID-19 (4). Even with the spread of the delta variant of the virus, both vaccines remain highly effective at preventing severe COVID-19 that results in hospital admission and death.
Mild side effects are common with all SARS-CoV-2 vaccines and reflect the immune system being stimulated by the vaccine. Severe side effects have been reported in a transparent manner during the trials and during programmatic use but are exceptionally rare. The overwhelming benefits of vaccination in terms of preventing death from COVID-19 far outweigh the risks of these rare severe side effects.
As a professional society, we therefore strongly recommend the use of vaccination for COVID-19 and would encourage all people (including transplant recipients) to be vaccinated and so help protect themselves and others.
References:
- M. Alfishawy et al. Int J Org Transplant Med 2020; Vol. 11 (4): p145-162
- Gupta G, Azhar A, Gungor A, Molnar MZ. Early Data on Utilization and Discard of Organs From COVID-19 – infected Donors: A US National Registry Analysis. 2022;00(00):19-21. doi:10.1097/TP.0000000000004091
- Polack, Fernando P., et al. "Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine." New England Journal of Medicine (2020).
- Sadoff, Jerald, et al. "Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19." New England Journal of Medicine 384.23 (2021): 2187-2201.
Kind regards,
Dr David Thomson - SATS President, on behalf of the EXECUTIVE COMMITTEE
11 July 2022
Dr David Thomson – SATS President, on behalf of the EXECUTIVE COMMITTEE
Blood Group Incompatible (ABOi) Transplantation
Statement of the South African Society of Transplantation
Date: 11 May 2023
Introduction and Context
There is a global organ shortage resulting in many patients dying on transplantation wait lists. This has resulted in the development of numerous alternative clinical techniques to increase the availability of donor organs. The use of blood group incompatible organs from both deceased donor and living donors is a well validated and safe method for improving both access to transplantation and the number of transplants to be performed. In the era of improved pharmacotherapies such as the availability of the CD-20 monoclonal antibody Rituximab, improvements in isoagglutinin control and testing, ABOi transplantation is safe and results in equivalent patient and graft survival when compared to blood group compatible transplantation.
Purpose of this statement
- To inform the circumstances and contexts under which ABOi transplantation can be considered
- To advise on best practice principles through which ABOi transplantation should be performed at transplant centres
- To propose a centralised, national platform for the development of treatment protocols, the collection of data and the development of new methodologies, techniques, and pharmacotherapies as they pertain to ABOi transplantation to ensure that a standardised and consistent baseline for care is established in South Africa
Context and Clinical Circumstances under which ABOi should be considered
ABOi transplantation can be considered in the following circumstances, provided there is informed consent from the patient or their surrogate, and a standardised institutional protocol for ABOi transplantation exists:
- In all solid organ recipients where there is the availability of a deceased donor ABOi organ, and when there is no better suited compatible recipient of the same blood group, and where the risk of denying access to transplantation is outweighed by the benefit to patient survival.
- In the acute organ failure setting where a viable living donor option exists, and there is no viable or available ABO compatible living donor or deceased donor, and where the risk of denying access to transplantation is outweighed by the benefit to patient survival.
- In the elective setting where there is no available ABO compatible living donor
- Where the recipient has a viable and available ABOi donor where the treating clinical team deems there to be no clinical contraindications to treatment and the option of a paired exchange has been explored.
These options should also be considered in children and adolescents in centres who have experience with this.
ABOi transplantation should not be considered at this time in the following situations:
- If the recipient has a viable and available deceased donor or living donor ABO compatible donor offer
- In the setting of donation after circulatory death (DCD)
- If the measured isoagglutinin titre using the dithiothreitol (DTT) treatment of plasma method is greater than 512 for the donor organ blood type
- If there is not informed consent
- If there is no standardised protocol for the management of ABOi transplantation at the institution offering the transplant
Protocol Development and Data Collection
- Institutions must develop institutionally contextualised and agreed upon protocols for the management of ABOi transplantation in their centre in line with accepted international standards
- Such protocols should be available for review
- We recommend the establishment of a National ABOi solid organ patient database for improving access and quality of ABOi transplantation for the South African population.
Best Practice Principals
- We recommend the use of a separate ABOi consenting process and form for recipients of ABOi organs which outlines the processes and risks associated with ABOi transplantation.
- We recommend the use of both PBS and Dithiothreitol (DTT) validated isoagglutinin testing for the estimation of IgG and IgM anti-A and anti-B antibody titres in recipient plasma samples.
- We recommend that institutional protocols utilise either plasmapheresis techniques and/or immunoadsorption columns for the pre- and post-operative control of isoagglutinin titres in recipients of ABOi solid organ transplants and that the safety and efficacy of the techniques in question must consider individual as well as institutional factors such as bleeding risk, haemodynamic compromise, cost and availability.
- We recommend that the protocolised care of ABOi recipients include the use of the CD20 monoclonal antibody therapy Rituximab.
- We do not recommend the routine use of splenectomy as a method to control isoagglutinin levels in recipients of ABOi solid organ transplantation.
- Immunosuppression regimens and immunomodulatory techniques and protocols must be adaptable to the individual patient requirements and treatment runs.
Conclusion
ABOi transplantation is a viable alternative for solid organ recipients and must be performed by experienced and well-trained clinical teams. The use of ABOi organs is safe and results in improved access to and availability of transplantation.
References
- Girlanda R. World Journal of Transplantation © 2016. 2016;6(3):451–60.
- Lee EC, Kim SH, Park S, Lee EC, Kim SH, Park S. Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis. 2017;23(35):6516–33.
- Yadav DK, Hua YF, Bai X, Lou J, Que R, Gao S, et al. Review Article ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. 2019;2019.
- Geyer M, Fischer KG, Drognitz O, Walz G, Pisarski P, Wilpert J. ABO-incompatible kidney transplantation with antigen-specific immunoadsorption and rituximab - Insights and uncertainties. Contrib Nephrol. 2009; 162:47–60.
- de Magnée C, Brunée L, Tambucci R, Pire A, Scheers I, Sokal EM et al. Is ABO-Incompatible Living Donor Liver Transplantation Really a Good Alternative for Pediatric Recipients? Children. 2021;8(7):600.
- Peruhova M, Georgieva V, Yurukova N, Sekulovska M, Panayotova G, Mihova A, et al. ABOnonidentical liver transplantation from a deceased donor and clinical outcomes following antibody rebound: A case report. World J Transplant. 2020;10(5):138–46.
Contact Information
Prof Mignon McCulloch
Tel: +27(0)827804097
Email: mignon.mcculloch@uct.ac.za
President of South African Transplant Association (SATS)